Informatics on High-Throughput Sequencing Data
CBW HT-seq Module 2 - IGV lab
This lab was created by Sorana Morrissy, then modified by Florence Cavalli
Introduction
Description of the lab
Welcome to the lab for Genome Visualization! This lab will introduce you to the Integrative Genomics Viewer, one of the most popular visualization tools for High Throughput Sequencing (HTS) data.
After this lab, you will be able to:
- visualize a variety of genomic data
- very quickly navigate around the genome
- visualize HTS read alignments
- validate SNP calls and structural re-arrangements by eye
Things to know before you start:
- The lab may take between 1-2 hours, depending on your familiarity with genome browsing. Don’t worry if you don’t complete the lab! It is available for you to complete later.
- There are a few thought-provoking Questions or Notes pertaining to sections of the lab. These are optional, and may take more time, but are meant to help you better understand the visualizations you are seeing. These questions will be denoted by boxes, as follows:
Question(s):
- Thought-provoking question goes here
Requirements
- Integrative Genomics Viewer
- Ability to run Java
Compatibility
This tutorial was intended for IGV v2.3, which is available on the Download page. It is strongly recommended that you use this version, as other versions may not be compatible. If you have installed a former version, please uninstall it and install the latest version.
Data Set for IGV
- Chromosome 21: 19,000,000-20,000,000
- HCC1143.normal.21.19M-20M.bam
- HCC1143.normal.21.19M-20M.bam.bai
Check the online student page
Your instructors may update the lab with clarifications or more bonus sections.
Visualization Part 1: Getting familiar with IGV
We will be visualizing read alignments using the Integrative Genomics Viewer, a popular visualization tool for HTS data.
First, lets familiarize ourselves with it.
Get familiar with the interface
Load a Genome and Data Tracks
By default, IGV loads Human hg19. If you work with another version of the human genome, or another organism altogether, you can change the genome by clicking the drop down menu in the upper-left. For this lab, we’ll be using Human hg19.
We will also load additional tracks from Server:
- Ensembl genes (or your favourite source of gene annotations)
- GC Percentage
- dbSNP 1.4.7
Note: If you are using a computer with low memory, only load the gene annotations.


Navigation
You should see listing of chromosomes in this reference genome. Choose 1, for chromosome 1.

Navigate to chr1:10,000-11,000 by entering this into the location field (in the top-left corner of the interface) and clicking Go. This shows a window of chromosome 1 that is 1,000 base pairs wide and beginning at position 10,000.
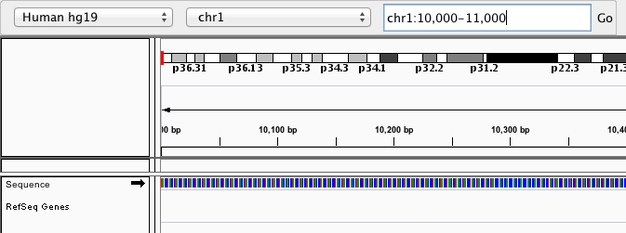
IGV displays the sequence of letters in a genome as a sequence of colours (e.g. A = green). This makes repetitive sequences, like the ones found at the start of this region, easy to identify.
You can navigate to a gene of interest by typing it in the same box the genomic coordinates are in and pressing Enter/Return. Try it for your favourite gene, or BRCA1 if you can’t decide.
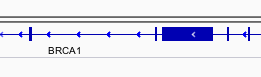
Genes are represented as lines and boxes. Lines represent intronic regions, and boxes represent exotic regions. The arrows indicate the strand on which the gene lies.
When loaded, tracks are stacked on top of each other. You can identify which track is which by consulting the label to the left of each track.
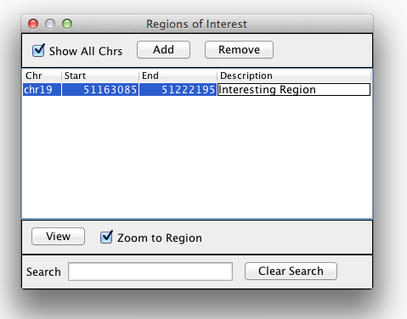
Region Lists
Sometimes, it’s really useful to save where you are, or to load regions of interest. For this purpose, there is A Region Navigator in IGV. To access it, click Regions > Region Navigator. While you browse around the genome, you can save some bookmarks by pressing the Add button at any time.
Loading Read Alignments
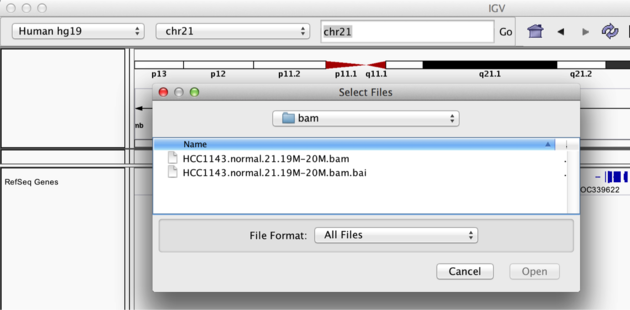
We will be using the breast cancer cell line HCC1143 to visualize alignments. For speed, only a small portion of chr21 will be loaded (19M:20M).
HCC1143 Alignments to hg19:
Copy the files to your local drive, and in IGV choose File > Load from File…, select the bam file, and click OK. Note that the bam and index files must be in the same directory for IGV to load these properly.
Visualizing read alignments
Navigate to a narrow window on chromosome 21: “chr21:19,480,041-19,480,386”.
To start our exploration, right click on the track-name, and select the following options:
- Sort alignments by “start location”
- Group alignments by “pair orientation”
Experiment with the various settings by right clicking the read alignment track and toggling the options. Think about which would be best for specific tasks (e.g. quality control, SNP calling, CNV finding).
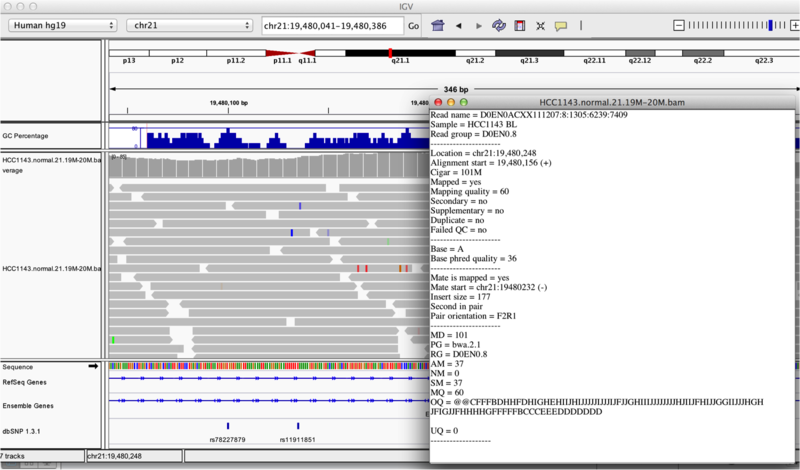
You will see reads represented by grey or white bars stacked on top of each other, where they were aligned to the reference genome. The reads are pointed to indicate their orientation (i.e. the strand on which they are mapped). Mouse over any read and notice that a lot of information is available. To toggle read display from “hover” to “click”, select the yellow box and change the setting.
Once you select a read, you will learn what many of these metrics mean, and how to use them to assess the quality of your datasets. At each base that the read sequence mismatches the reference, the colour of the base represents the letter that exists in the read (using the same colour legend used for displaying the reference).
Visualization Part 2: Inspecting SNPs, SNVs, and SVs
In this section we will be looking in detail at 8 positions in the genome, and determining whether they represent real events or artifacts.
Neighbouring somatic SNV and germline SNP
Navigate to position “chr21:19,479,237-19,479,814”
- center on the SNV, sort by base (window “chr21:19,478,749-19,479,891” is centered on the SNV)
- Sort alignments by “base”
- Color alignments by “read strand”
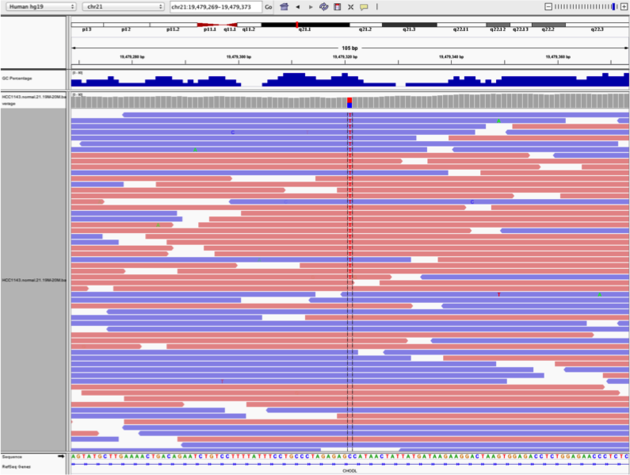
Notes:
- High base qualities in all reads except one (where the alt allele is the last base of the read)
- Good mapping quality of reads, no strand bias, allele frequency consistent with heterozygous mutation
Question(s):
- What does Shade base by quality do? How might this be helpful?
- How does Color by “read strand” help?
Homopolymer repeat with indel
Navigate to position “chr21:19,518,412-19,518,497”
- Group alignments by “read strand”
- Center on the second “T”, and Sort alignments by “base” on the forward strand reads
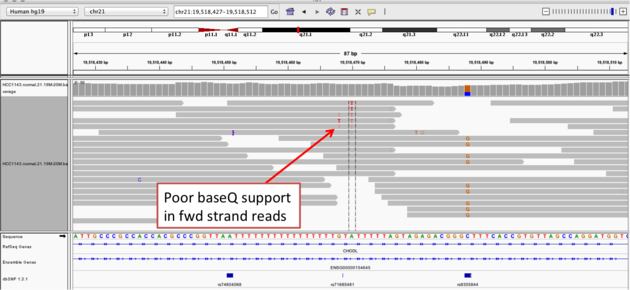
- center on the one base deletion, and Sort alignments by “base” on the reverse strand reads
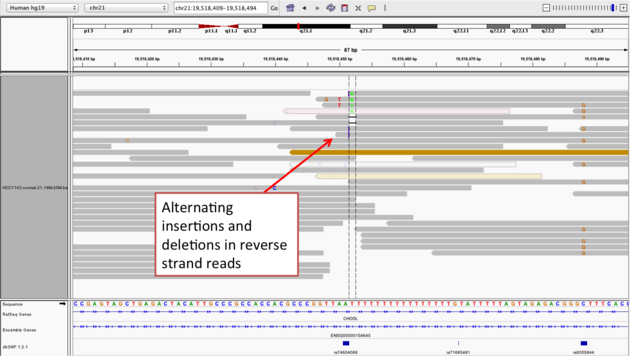
Notes:
- The alt allele is either a deletion or insertion of one or two “T”s
- The remaining bases are mismatched, because the alignment is now out of sync
- (Using an older version of dbSNP (1.3.1) the entry at this location (rs74604068) is an A->T, and in all likelihood an artifact i.e. the common variants included some cases that are actually common misalignments caused by repeats. This is getting better; this entry is not present anymore in dbSNP 1.4.7!)
Coverage by GC
Navigate to position “chr21:19,611,925-19,631,555”
Note that the range contains areas where coverage drops to zero in a few places.
- use Collapsed view
- Color alignments by “insert size”
- load GC track (if not loaded already)
- see concordance of coverage with GC content
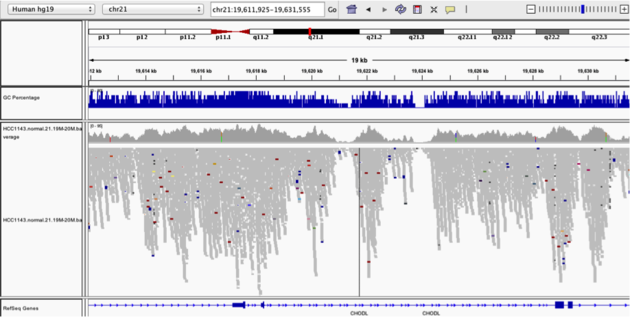
Question:
- Why are there blue and red reads throughout the alignments?
Heterozygous SNPs on different alleles
Navigate to region “chr21:19,666,833-19,667,007”
- sort by base
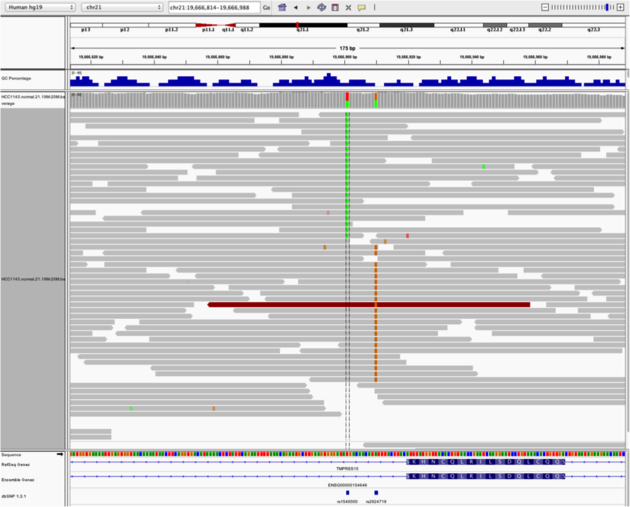
Note:
- Linkage between alleles is obvious in this case because both are spanned by the same reads
Low mapping quality
Navigate to region “chr21:19,800,320-19,818,162”
- use Collapsed view
- load repeat track

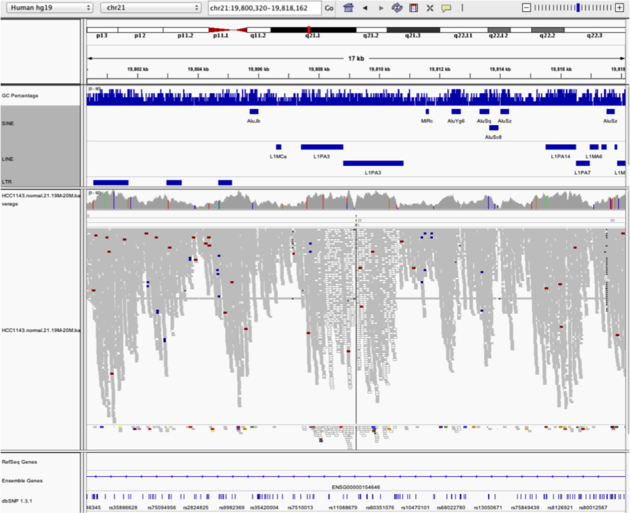
Notes:
- Mapping quality plunges in all reads (white instead of grey). Once we load repeat elements, we see that
- : there are two LINE elements that cause this.
Homozygous deletion
Navigate to region “chr21:19,324,469-19,331,468”
- sort reads by insert size
- turn on “View as Pairs” and “Expanded” view
- click on a red read pair to pull up information on alignments
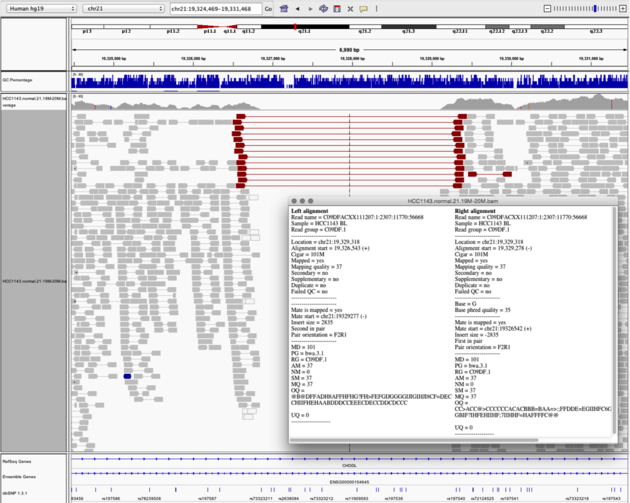
Notes:
- Typical insert size of read pair in the vicinity: 350bp
- New insert size of red read pairs: 2,875bp
- This corresponds to a homozygous deletion of 2.5kb
Mis-alignment
Navigate to region “chr21:19,102,154-19,103,108”
- turn on “View as Pairs” and “Expanded” view
- Group alignments by “Pair orientation”
- Color alignments by “Insert size and pair orientation”

Notes:
- This is a position where AluY element causes mis-alignment.
- Misaligned reads have mismatches to the reference and
- Well-aligned reads have partners on other chromosomes where additional AluY elements are encoded.
Translocation
Navigate to region “chr21:19,089,694-19,095,362”
- Expanded view
- Group by “Pair orientation”
- Color alignments by “Insert size and pair orientation”
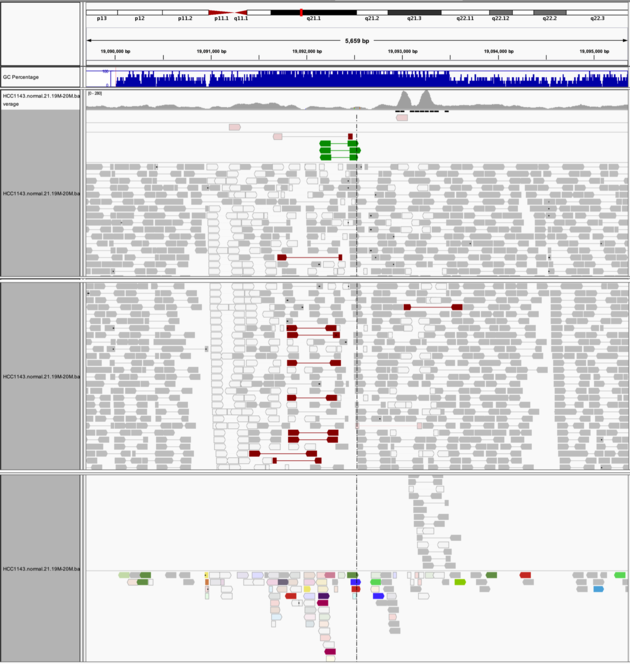
Notes:
- many reads with mismatches to reference
- read pairs in RL pattern (instead of LR pattern)
- region is flanked by reads with poor mapping quality (white instead of grey)
- presence of reads with pairs on other chromosomes (coloured reads at the bottom when scrolling down)
Optional- Visualization Part 3: Automating Tasks in IGV
We can use the Tools menu to invoke running a batch script. Using a batch script, you can automatically load your data, go to a particular location, set some display options and take a snapshot. This can be useful when you want to inspect many variant calls.
Batch scripts are described on the IGV website: batch file requirements commands recognized in a batch script
We also need to provide sample attribute file as described here
Download the batch script and the attribute file for our dataset:
- batch script: Run_batch_IGV_snapshots_example.txt here
- attribute file: igv_HCC1143_attributes.txt here
After downloading those two files, please update the paths for the bam file and the output directory (indicated as **** in the file) in the batch script to set your “local paths”, with a text editor.
Now run the file from the Tools menu:
Tools -> Run Batch Script
The igv screenshots are in the screenshots output directory you set! Have a look!
You’re done! We hope that you enjoyed the lab and that you continue to enjoy IGV.